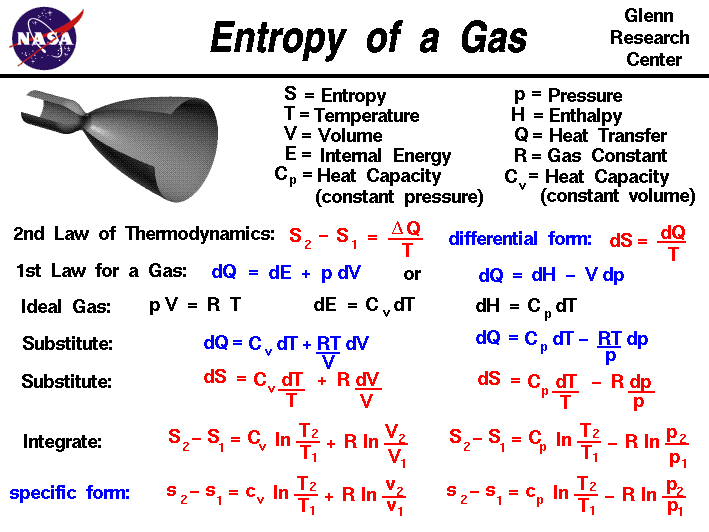
Thermodynamics is a branch of physics
that deals with the energy and work of a system. In aerodynamics,
we are most interested in thermodynamics in the study of propulsion
systems and understanding high speed
flows. The first law of thermodynamics
indicates that the total energy of a system is conserved. Total energy
includes the potential and kinetic energy, the
work
done by the system, and the transfer of
heat
through the system. The
second law of thermodynamics indicates
that, while many physical processes that satisfy the first law are
possible, the only processes that occur in nature are those for which
the entropy of the system either remains constant or
increases.
Entropy, like temperature and
pressure, can be explained on both a
macro scale and a
micro scale.
Since thermodynamics deals only with the macro scale, the
change in entropy delta S is defined
here to be the heat transfer delta Q into the system divided by the
temperature T:
delta S = delta Q / T
During a thermodynamic process, the temperature T of an object
changes as heat Q is applied or extracted. A more correct definition of
the entropy S is the differential form that accounts for this
variation.
dS = dQ / T
The change in entropy is then the inverse
of the temperature integrated over the change in heat transfer. For
gases, there are two possible ways to evaluate the change in entropy.
We begin by using the first law of thermodynamics:
dE = dQ - dW
where E is the internal energy and W is the work done by
the system.
Substituting for the definition of work
for a gas.
dQ = dE + p dV
where p is the
pressure
and V is the
volume of the gas. If we use the
definition of the
enthalpy H
of a gas:
H = E + p * V
Then:
dH = dE + p dV + V dp
Substitute into the first law equation:
dQ = dH - V dp - p dV + p dV
dQ = dH - V dp
is an alternate way to present the first law of thermodynamics.
For an ideal gas, the
equation of state is written:
p * V = R * T
where R is the gas constant.
The heat transfer of a
gas is equal to the heat capacity times the change in
temperature; in differential form:
dQ = C * dT
If we have a constant volume process,
the formulation of the first law gives:
dE = dQ = C (constant volume) * dT
Similarly, for a constant pressure process,
the formulation of the first law gives:
dH = dQ = C (constant pressure) * dT
If we assume that the heat capacity is constant with temperature,
we can use these two equations to define the change in enthalpy
and internal energy. If we substitute the value for
p from the equation of state, and the definition of
dE in the first energy equation, we obtain:
dQ = C (constant volume) * dT + R * T dV / V
Similarly substituting the value of V from the equation of
state, and the definition of dH we obtain the alternate form:
dQ = C (constant pressure) * dT - R * T dp / p
Substituting these forms for dQ into the differential form
of the entropy equation gives::
dS = C (constant volume) * dT / T + R * dV / V
and
dS = C (constant pressure) * dT / T - R * dp / p
These equations can be integrated from condition "1" to
condition "2" to give:
S2 - S1 = Cv * ln ( T2 / T1) + R * ln ( V2 / V1)
and
S2 - S1 = Cp * ln ( T2 / T1) - R * ln ( p2 / p1)
where Cv is the heat capacity
at constant volume,
Cp is the heat capacity
at constant pressure,
and ln is the symbol for the
logarithmic function.
If we divide both equations by the mass of gas, we can obtain
intrinsic, or
"specific"
forms of both equations:
s2 - s1 = cv * ln ( T2 / T1) + R * ln ( v2 / v1)
and
s2 - s1 = cp * ln ( T2 / T1) - R * ln ( p2 / p1)
where cp and cv are the
specific heat capacities.
Depending on the type of process we encounter, we can now determine
the change in entropy for a gas.
These equations can be a bit confusing, because we use the specific heat at
constant volume when we have a process that changes volume, and the specific
heat at constant pressure when the process changes pressure.To clarify matters,
let's look at the first equation:
s2 - s1 = cv * ln ( T2 / T1) + R * ln ( v2 / v1)
If we have a constant volume process, the second term in the equation is equal
to zero, since v2/v1 = 1. We can then determine the value of the specific heat
for the constant volume process. But if we have a process that changes volume, the
second term in the equation is not zero. We can think of the first term of the equation
as the contribution for a constant volume process, and the second term as the additional
change produced by the change in volume. A similar type of argument can be made
for the equation used for a change in pressure.
Activities:
Guided Tours
-
 Thermodynamics:
Thermodynamics:

-
 Compressor:
Compressor:

-
 Power Turbine:
Power Turbine:

Navigation ..


- Beginner's Guide Home Page
|
