Thermodynamics is a branch of physics
which deals with the energy and work of a system.
Thermodynamics deals only with the
large scale response
of a system which we can observe
and measure in experiments. Small scale gas interactions are
described by the kinetic theory of gases.
There are three principal
laws of thermodynamics which are described on separate slides. Each
law leads to the definition of
thermodynamic properties
which help us to understand and predict the operation of a physical
system. We will present some simple examples of these laws and
properties for a variety of physical systems, although
we are most interested in the thermodynamics of
propulsion systems
and
high speed flows.
Fortunately, many of the
classical examples of thermodynamics involve gas dynamics.
In our observations of the work done on,
or by a gas, we have found that the amount of work depends not only
on the initial and final states of the gas
but also on the process, or path which produces the final state.
Similarly the amount of heat transferred into, or
from a gas also depends on the initial and final states and the
process which produces the final state. Many observations of real
gases have shown that the difference of the heat flow into the gas
and the work done by the gas depends only on the initial and final
states of the gas and does not depend on the process or path
which produces the final state. This suggests the existence of an
additional variable, called the internal energy of the gas,
which depends only on the state of the gas and not on any process.
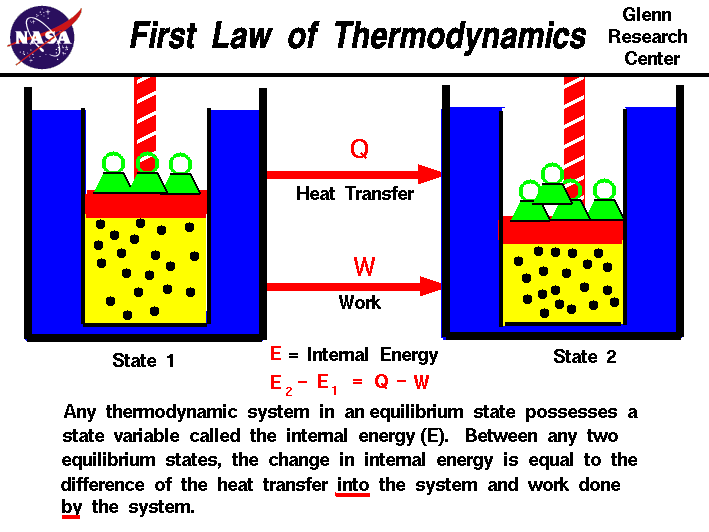
The internal energy is a state variable, just like the temperature or
the pressure. The first law of thermodynamics defines the internal
energy (E) as equal to the difference of the heat transfer (Q) into
a system and the work (W) done by the system.
E2 - E1 = Q - W
We have emphasized the
words "into" and "by" in the definition. Heat removed from a system
would be assigned a negative sign in the equation. Similarly work
done on the system is assigned a negative sign.
The internal energy is just a form of energy like the potential
energy of an object at some height above the earth, or the kinetic
energy of an object in motion. In the same way that potential energy
can be converted to kinetic energy while conserving the total energy
of the system, the internal energy of a thermodynamic system can be
converted to either kinetic or potential energy. Like potential
energy, the internal energy can be stored in the system.
Notice, however, that heat and
work can not be stored or conserved independently since they depend
on the process. The first law of thermodynamics allows for many
possible states of a system to exist, but only certain states are
found to exist in nature. The
second law
of thermodynamics helps to
explain this observation.
If a system is fully insulated from the outside environment, it is
possible to have a change of state in which no heat is transferred into the
system. Scientists refer to a process which does not involve heat
transfer as an adiabatic process.
The implementation
of the first law of thermodynamics for gases introduces another
useful state variable called the enthalpy
which is described on a separate page.
Activities:
Guided Tours
-
 Thermodynamics:
Thermodynamics:

Navigation ..


- Beginner's Guide Home Page
|
