| Antacid
Tablet Race
TOPIC:
Rocket fuels and propellants
Objective: To
demonstrate how increasing the surface area of a chemical increases its
reaction rate. Description:
A whole antacid tablet and a crushed tablet are added to separate
beakers of water so that their relative reaction rates can be compared.
EDITED BY:
Roger Storm, NASA Glenn Research Center
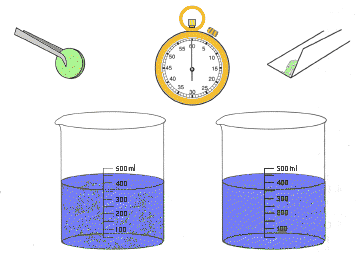 Materials: Materials:
- Antacid tablets
(two per test)
- Two beakers (or
glass or plastic jars)
- Tweezers or forceps
- Scrap paper
- Watch or clock
with second hand
- Small block of
wood
Procedure:
- Fill both beakers
about half full with water of the same temperature.
- Wrap paper around
one antacid tablet. Place the packet on a hard surface and crush the
tablet by pressing on it with the wood block.
- Open the paper
packet with the crushed tablet and hold it over one of the beakers.
Pour the powder in the water and time how long it takes for the powder
to dissolve.
- Pick up a whole
tablet and drop it into the second beaker of water. Time how long it
takes to dissolve completely.
Discussion:
This activity demonstrates how increasing the surface area of an antacid
tablet by crushing it into a powder increases the rate in which it dissolves
in water. This is a similar situation to the way the thrust of a rocket
is increased by increasing the burning surface of its propellants. Increasing
the burning surface increases its burning rate since more fuel becomes
exposed to oxygen . In solid rockets, a hollow core extending the length
of the propellant will permit more propellant to burn at a time. This
increases the acceleration of the gases produced as they leave the rocket
engine. Liquid propellants are sprayed into the combustion chamber of
a liquid propellant rocket to increase their surface area. Smaller droplets
react more quickly than do large ones, increasing the acceleration of
the escaping gases.
Teaching Notes and
Questions:
- This activity
is an ideal way for safely showing how the burning rate of rocket propellants
is increased without having the students use fire.
- A similar activity
can be tried with small pieces of hard candy. Take two pieces of candy
and crush one. Then, give the whole candy piece to one student and the
crushed candy to another student to dissolve in their mouths. Which
candy will dissolve first?
- Demonstrate the
same effect by trying to ignite a thick piece of wood with a match.
Next, cut the wood with a sharp knife to make shavings. Then, try to
ignite the shavings. Caution: Be sure to exercise proper safety precautions
with fire.
| 