Thermodynamics is a branch of physics
which deals with the energy and work of a system.
Thermodynamics
deals only with the
large scale response
of a system which we can
observe and measure in experiments. In aerodynamics, we are most
interested in thermodynamics for the role it plays in
engine design
and
high speed flight.
On this slide we derive some equations which relate the heat capacity
of a gas to the gas constant used in the
equation of state.
We are going to be using
specific
values of the state variables.
For a scientist, a "specific" state variable means the value of the variable
divided by the mass of the substance. This allows us to derive relations between
variables without regard for the amount of the substance that we have. We can
multiply the specific variable by the quantity of the substance at any time
to determine the actual value of the flow variable.
From our studies of
heat transfer,
we know that the amount of heat transferred between two objects is
proportional to the
temperature
difference between the objects and the
heat capacity of the objects.
The heat capacity is a
constant that tells how much heat is added per unit temperature rise.
The value of the constant is different for different materials and depends
on the process. Heat capacity is not a state variable.
If we are dealing with a gas, it is most convenient to use forms of the
thermodynamics equations based on the
enthalpy
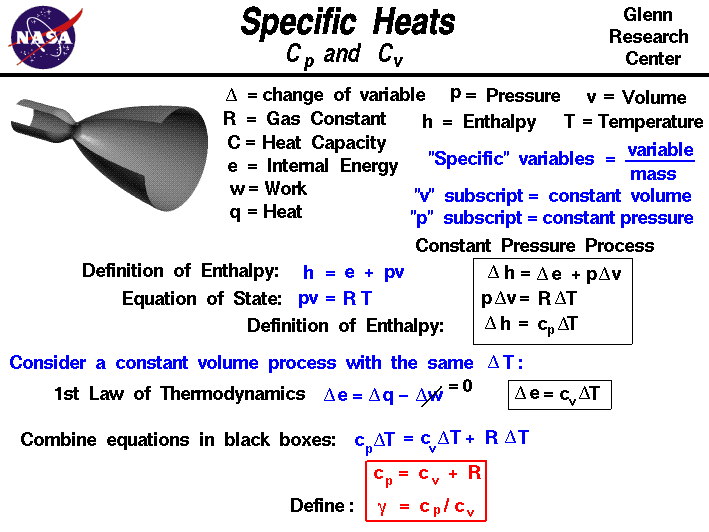
of the gas. From the definition of enthalpy:
h = e + p * v
where h in the specific enthalpy, p is the
pressure,
v is the
specific volume,
and e is the specific
internal energy.
During a process, the values of these variables change. Let's denote
the change by the Greek letter delta which looks like a triangle.
So "delta h" means the change of "h" from state 1 to state 2 during a process.
Then, for a constant pressure process the
enthalpy equation becomes:
delta h = delta e + p * delta v
The enthalpy, internal energy, and volume are all changed,
but the pressure remains the same.
From our derivation of the
enthalpy equation,
the change of specific enthalpy is equal to the heat transfer for a constant pressure process:
delta h = cp * delta T
where delta T is the change of temperature of the gas during the process,and c is the specific heat capacity.
We have added a subscript "p" to the specific heat capacity to remind us that this value
only applies to a constant pressure process.
The equation of state of a gas relates the temperature,
pressure, and volume through a gas constant R . The gas constant used by
aerodynamicists is derived from the universal gas constant, but has a unique value
for every gas.
p * v = R * T
If we have a constant pressure process, then:
p * delta v = R * delta T
Now let us imagine that we have a constant volume process with our gas that
produces exactly the same temperature change as the constant pressure process that
we have been discussing. Then the
first law of thermodynamics tells us:
delta e = delta q - delta w
where q is the specific heat transfer and w is the work done by
the gas. For a constant volume process, the
work
is equal to zero. And we can express the
heat transfer as a constant times the change in temperature.
This gives:
delta e = cv * delta T
where delta T is the change of temperature of the gas during the process,and c is the specific heat capacity.
We have added a subscript "v" to the specific heat capacity to remind us that this value
only applies to a constant volume process.
Even though the temperature change is the same for this process and the constant
pressure process, the value of the specific heat capacity is different.
Because we have selected the constant volume process to give the same change in
temperature as our constant pressure process, we can substitute the expression
given above for "delta e" into the enthalpy equation. In general, you can't
make this substitution because a constant pressure process and a constant volume process
produce different changes in temperature
If we substitute the expressions for "delta e", "p * delta v", and "delta h" into
the enthalpy equation we obtain:
cp * delta T = cv * delta T + R * delta T
dividing by "delta T" gives the relation:
cp = cv + R
The specific heat constants for constant pressure and constant volume processes
are related to the gas constant for a given gas. This rather remarkable result
has been derived from thermodynamic relations, which are based on observations
of physical systems and processes. Using the
kinetic theory
of gases, this same result can be derived
from considerations of the conservation of energy at a molecular level.
We can define an additional
variable called the specific heat
ratio,
which is given the Greek
symbol "gamma", which is equal to cp divided by cv:
gamma = cp / cv
"Gamma" is just a number whose value depends on the state of the gas. For air,
gamma = 1.4 for standard day conditions. "Gamma" appears in many fluids equations
including the equation relating pressure, temperature, and volume during a
simple compression or expansion
process, the equation for the
speed of sound,
and all the equations for
isentropic flows,
and
shock waves.
Because the value of
"gamma" just depends on the state of the gas, there are tables of these values
for given gases. You can use the tables to solve gas dynamics problems.
Activities:
Guided Tours
Navigation..


- Beginner's Guide Home Page
|
