|
Beginner's
Guide to Propulsion
Boyle's Law
Answers
Answers
will vary. Suggested answers are shown below:
1. Which variable
is plotted on the graph's vertical axis? Pressure
2. Which variable
is plotted on the graph's horizontal axis? Volume
3.Locate the temperature
gauge. What is the Kelvin temperature? Close
to 300 K
4.Which of the following
conditions is that temperature closer to? room temperature? human body
temperature? freezer temperature? Room temperature
5.The red plunger
is used to exert pressure on the gas molecules in which colored area ?
Yellow
6.Complete the table
below as you watch the animated gas lab.
|
Pressure
|
VOLUME
|
|
1.00
|
4
|
|
1.33
|
3
|
|
2.00
|
2
|
7.What do you predict
the volume will be when the pressure becomes 4.00 ? 1
8.Sketch the completed
pressure-volume graph below:
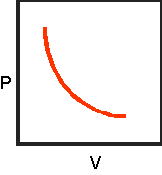
9.Click on "Effect
of Changing Pressure on Volume." Describe what is added to the piston
to increase the pressure. Green
masses are added.
10.Sketch the completed
volume-pressure graph below:
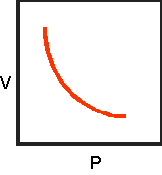
11.Write the formula
equation for Boyle's Law. PV = C
12.Write the equation
for Boyle's Law in words. The pressure
of a gas times its volume is a constant.
13.In the animated
gas lab, the units of pressure are Kilopascals
14.What are the units
of volume used in this lab? Cubic meters
15.Predict what the
volume in this lab would be if the pressure were 8.00. 0.5
16.Predict what the
volume in this lab would be if the pressure were 0.500. 8
17.State Boyle's law
in your own words: When the pressure on a
gas in a closed container is increased, its volume is decreased. The volume
of a gas will increase if the pressure is lowered.
18.Describe what happens
to the pressure of the air in the bag as you decrease its volume. The
air in the bag exerts more pressure.
19.How does your experience
with the plastic bag compare to the animated gas lab? In
both cases, increasing pressure results when the volume of the gas is
reduced.
20.If, in either situation,
instead of having a closed container you had a small opening in the bottom
of it, what would eventually happen to the gas? The
gas would escape through the opening.
21.Refer to the previous
question. What would happen to the volume of the gas in
the container? It would decrease until all
of the air was pushed out of the bag.
22.Refer to the previous
questions. What would happen to the pressure of the gas?
It would also decrease because there eventually
would be no air left to exert any pressure on the bag.
23.Based on the questions
above, hypothesize why a jet engine must constantly have air flowing into
it in order to maintain pressure. Since a
jet engine is not a closed container, air must constantly flow in to replace
the air used in combustion and leaving the nozzle.
|
