To move an airplane through the air,
thrust is generated by some kind of
propulsion system.
Beginning with the Wright brothers'
first flight,
many airplanes have used
internal combustion engines
to turn
propellers
to generate thrust.
Today, most general aviation or private airplanes are
powered by internal combustion (IC) engines, much like
the engine in your family automobile.
When discussing engines, we must consider both the
mechanical operation of the
machine and the
thermodynamic
processes that enable the machine to produce useful
work.
On this page we consider the thermodynamics of a
four-stroke
IC engine.
To understand how a propulsion system works, we must
study the basic thermodynamics of
gases.
Gases have various
properties that we can observe with our
senses, including the gas
pressure p,
temperature T,
mass, and
volume V
that contains the gas.
Careful, scientific observation has determined that these
variables
are related to one another, and the values of these
properties determine the
state
of the gas.
A thermodynamic process, such as
heating or
compressing the gas,
changes the values of the state variables in a
manner which is described by the
laws of thermodynamics. The
work done by a gas
and the
heat transferred to a gas
depend on the beginning and ending states of the gas and
on the process used to change the state.
It is possible to perform a series of processes, in which the state
is changed during each process, but the gas eventually
returns to its original state. Such a series of processes is
called a
cycle
and forms the basis for understanding engine operation.
On this page we discuss
the Otto Thermodynamic Cycle which is used in
all internal combustion engines.
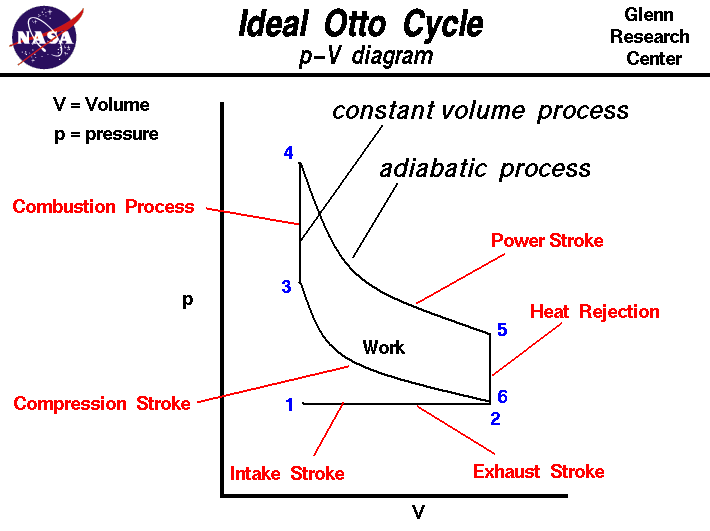
The figure shows a
p-V diagram
of the Otto cycle. Using the engine
stage numbering system,
we begin at the lower left with Stage 1 being the beginning of the
intake stroke of the engine. The pressure is near
atmospheric pressure and the gas volume is at a minimum.
Between Stage 1 and Stage 2 the piston is pulled out of the cylinder with
the intake valve open.
The pressure remains constant, and the gas volume increases
as fuel/air mixture is drawn into the cylinder through the intake valve.
Stage 2 begins the
compression stroke of the engine with the
closing of the intake valve. Between Stage 2
and Stage 3, the piston moves back into the cylinder, the gas volume decreases,
and the pressure increases because
work is done
on the gas by the piston. Stage 3 is the beginning of the
combustion
of the fuel/air mixture. The combustion occurs very quickly and the volume
remains constant.
Heat
is released during combustion which increases both the
temperature
and the pressure, according to the
equation of state.
Stage 4 begins the
power stroke of the engine.
Between Stage 4 and Stage 5,
the piston is driven towards the crankshaft,
the volume in increased, and the pressure
falls as
work is done
by the gas on the piston. At Stage 5 the exhaust valve is opened
and the residual heat in the gas is
exchanged
with the surroundings. The volume
remains constant and the pressure adjusts back to atmospheric conditions.
Stage 6 begins the
exhaust stroke of the engine during which the
piston moves back into the cylinder, the volume decreases and the pressure
remains constant. At the end of the exhaust stroke, conditions have returned
to Stage 1 and the process repeats itself.
During the cycle,
work
is done on the gas by the piston between stages 2 and 3. Work is done by
the gas on the piston between stages 4 and 5. The difference between the work done by the
gas and the work done on the gas is the area enclosed by the
cycle curve and is the work produced
by the cycle. The work times the rate of the cycle (cycles per second) is
equal to the
power
produced by the engine.
The area enclosed by the cycle on a p-V diagram
is proportional to the work produced by the cycle. On this page we have
shown an ideal Otto cycle in which there is no heat entering (or
leaving) the gas during the compression and power strokes, no friction
losses, and instantaneous burning occurring at constant volume. In reality,
the ideal cycle does not occur and there are many losses associated with
each process. These losses are normally accounted for by efficiency factors
which multiply and modify the ideal result. For a real cycle, the shape
of the p-V diagram is similar to the ideal, but the area (work) is
always less than the ideal value.
Activities:
Guided Tours
Navigation ..


- Beginner's Guide Home Page
|
